Have you ever stared at a chemical equation, feeling a mixture of confusion and intimidation? Wondering if the mysteries of molecular structure and chemical reactions will ever make sense? Fear not, fellow chemistry student! This comprehensive guide will equip you with the knowledge and tools to conquer Chemistry Unit 2 with confidence. We’ll delve into the intricacies of chemical bonds, explore the fascinating world of chemical reactions, and provide you with the answer key to ace that crucial review.

Image: www.studocu.com
Chemistry Unit 2 often sets the stage for a deeper understanding of the world around us. It lays the foundation for everything from understanding the processes that occur in our bodies to comprehending the complexities of environmental science. Knowing how to identify and analyze different types of chemical bonds, for example, unlocks the secrets of what makes materials strong, flexible, or even reactive. By mastering the principles of chemical reactions, we can grasp why certain substances combine to form new compounds, while others remain stubbornly separate. This knowledge is not just confined to the textbooks; it permeates our daily lives, influencing everything from the food we eat to the medicines we take.
Deciphering the Language of Chemical Bonding
The very essence of chemistry lies in how atoms interact with each other. These interactions are not just random collisions, but rather intricate dances governed by the fundamental laws of nature. Chemical bonding, the way atoms link together to form molecules, is the language that describes these dances.
Types of Bonds
Imagine atoms as tiny magnets, each with a positive and negative side. The way these tiny magnets interact determines the type of bond formed. The strongest bonds, *ionic bonds*, form when one atom completely transfers an electron to another, creating oppositely charged ions that attract each other. Sodium chloride (NaCl), commonly known as table salt, is a prime example of an ionic compound.
In contrast, *covalent bonds* involve atoms sharing electrons. This sharing can be equal, resulting in a *nonpolar covalent bond*, as seen in the oxygen molecule (O2), or unequal, resulting in a *polar covalent bond*, as in water (H2O). These subtle differences in electron sharing are what determine the properties of molecules and the reactions they participate in.
Bonding Theories
To understand the intricate dance of bonding, we delve into theories that explain the forces holding atoms together. Lewis theory provides a simple way to visualize bonds by depicting valence electrons, those involved in bonding, using dots. Valence Bond Theory takes a more detailed approach, describing bonds as the overlap of atomic orbitals – regions where electrons are likely to be found. Finally, Molecular Orbital Theory considers the formation of new orbitals when atoms combine, providing a more comprehensive understanding of molecular bonding.
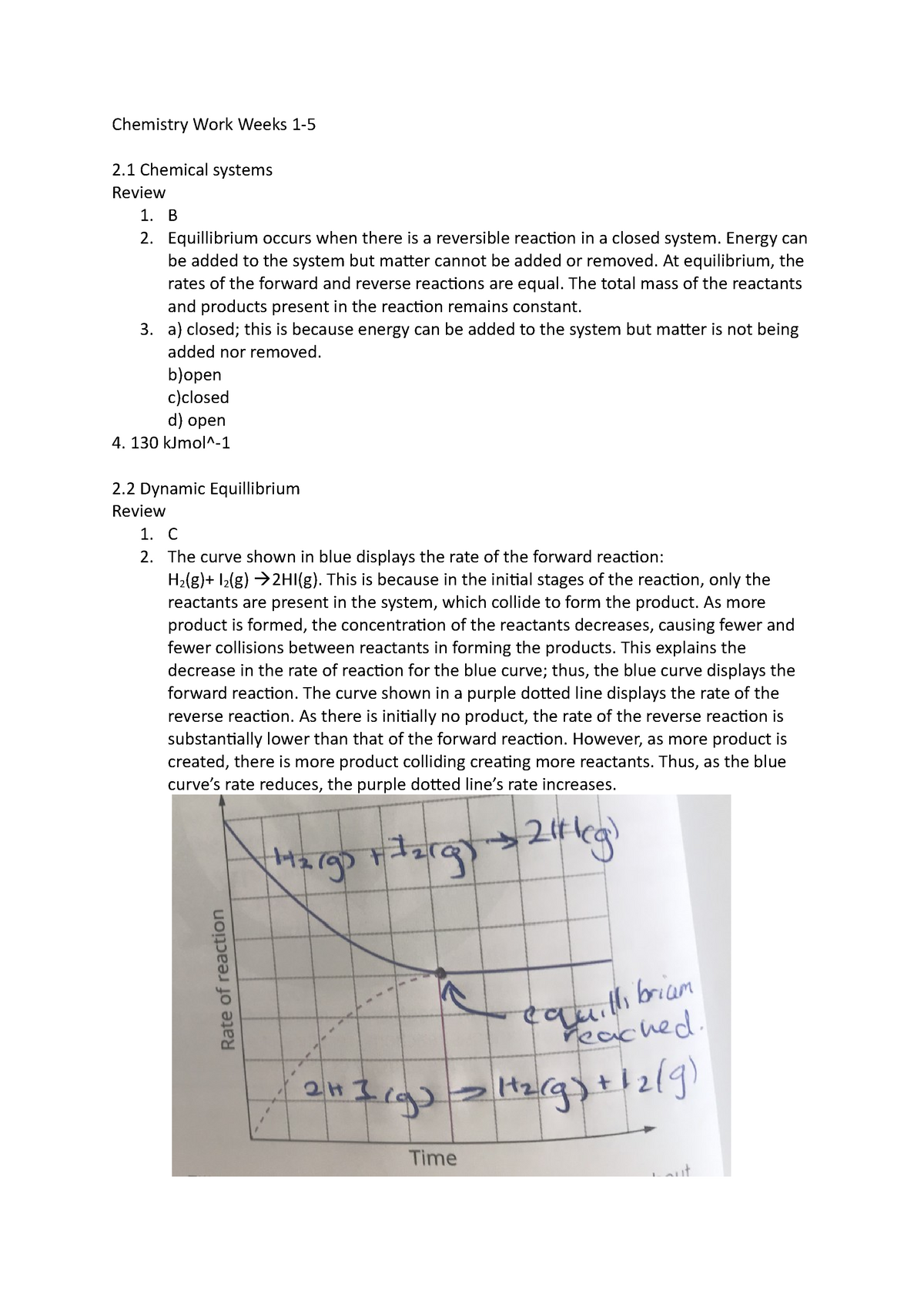
Image: www.studocu.com
Mapping the Landscape of Chemical Reactions
Just as molecules form through bonding, they can also break apart and recombine to form new molecules. These molecular transformations are what we call chemical reactions. Understanding the driving forces behind these changes is key to unlocking the secrets of chemistry.
Reaction Types
Chemical reactions can be classified based on the changes they involve. *Combination reactions* involve multiple reactants combining to form a single product, like the synthesis of water from hydrogen and oxygen. *Decomposition reactions* are the reverse, breaking down a single reactant into multiple products, as seen in the decomposition of calcium carbonate into calcium oxide and carbon dioxide. *Single replacement reactions* involve one element replacing another in a compound, while *double replacement reactions* exchange partners between two compounds.
Reaction Rates
Not all reactions occur at the same pace. Some, like the combustion of a match, happen quickly, while others, like the rusting of iron, occur over much longer timescales. Reaction rates describe the speed of a chemical reaction, influenced by factors like temperature, concentration of reactants, and the presence of catalysts.
Equilibrium
Chemical reactions often don’t proceed to completion; instead, they reach a state of equilibrium, where the rates of the forward and reverse reactions are equal. This dynamic balance is crucial for understanding the behavior of systems in nature, influencing everything from the pH of our blood to the composition of the atmosphere.
Navigating the Chemical Landscape: Practical Applications
The principles of chemical bonding and reactions are not confined to the pages of textbooks. They have profound applications in various fields, shaping the world we live in.
Medicine and Pharmaceuticals
Understanding how molecules interact is crucial in developing new drugs and treatments. By studying the shapes and reactivity of molecules, scientists can design drugs that target specific biological pathways, leading to novel therapies for diseases.
Materials Science
Chemical bonding determines the properties of materials. For example, the strong covalent bonds in diamonds make them incredibly hard, while the weaker bonds in plastics allow them to be molded and shaped. Understanding these bonds allows scientists to design new materials with specific desired properties.
Environmental Science
Chemistry plays a vital role in environmental protection. Understanding chemical reactions can help us analyze the impact of pollutants on the environment and develop strategies for remediation.
Mastering the Art of Chemistry Unit 2: Your Guide to Success
This guide has provided you with a comprehensive overview of Chemistry Unit 2, equipping you with the knowledge and tools to navigate its intricacies. Now, it’s time to put your knowledge to the test!
The Review Answer Key
We understand that practice is essential for mastery. To aid you in your review, we’ve compiled a comprehensive answer key for common Chemistry Unit 2 concepts. This key will provide you with valuable insights into the correct answers to practice questions, allowing you to identify any areas needing further reinforcement.
Chemistry Unit 2 Review Answer Key Pdf
Beyond the Basics
As you delve deeper into Chemistry Unit 2, remember that the beauty of this field lies in its endless possibilities. The concepts you’re learning lay the foundation for understanding a vast array of phenomena, from the intricacies of life to the wonders of the cosmos. Don’t hesitate to explore these connections, ask questions, and embark on your own journey of scientific inquiry.
Remember, mastering Chemistry Unit 2 is not just about memorizing facts but about developing a deeper understanding of the fundamental principles. This understanding will not only help you excel in your studies but also equip you with the knowledge and skills to navigate the complexities of the world around you. Continue your exploration, ask questions, and most importantly, enjoy the exciting journey of unraveling the secrets of chemistry.




