Have you ever wondered why some elements are bigger than others? Or why certain elements react more readily than others? The answer lies in the fascinating world of atomic radius, a key concept in chemistry that governs the size of atoms and plays a crucial role in determining their chemical properties. In this comprehensive guide, we will delve into the intricacies of atomic radius, explore the periodic trends that dictate its behavior, and provide you with the answers to common worksheet questions.

Image: studylistdavison99.s3-website-us-east-1.amazonaws.com
Understanding atomic radius is essential for comprehending the fundamental principles of chemistry. It allows us to predict how atoms will interact with each other, how they will form bonds, and how they will behave in various chemical reactions. By grasping the concept of atomic radius and its variations across the periodic table, we can unlock a deeper understanding of the chemical properties of different elements and their significance in the world around us.
Delving into the Basics: What is Atomic Radius?
The atomic radius is a measure of the size of an atom. It is defined as half the distance between the nuclei of two identical atoms that are bonded together. In simpler terms, it’s like measuring the diameter of an atom. However, determining the exact size of an atom is a complex task, as it lacks a defined boundary and its electrons are constantly moving around the nucleus.
Types of Atomic Radius
There are two primary types of atomic radius:
- Covalent radius: This refers to the distance between the nuclei of two atoms that are joined together by a covalent bond. It is typically measured in picometers (pm) or angstroms (Å).
- Metallic radius: This refers to the distance between the nuclei of two adjacent atoms in a metallic crystal lattice. It is also typically measured in pm or Å.
Periodic Trends: The Dance of Atomic Radius Across the Table
The periodic table is not just a jumbled arrangement of elements; it’s a carefully organized blueprint that reveals the relationship between atomic structure and chemical behavior. One of the most important periodic trends is the variation in atomic radius across the table. There are two key factors that influence this trend:
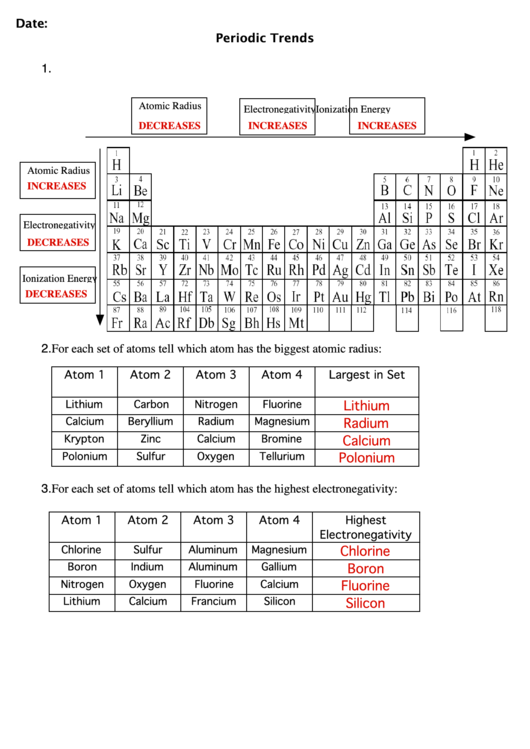
Image: www.formsbank.com
1. Across a Period (From Left to Right):
As we move from left to right across a period, the atomic radius generally decreases. This trend arises from the increasing number of protons and electrons in the atom. With more protons in the nucleus, the positive charge increases, attracting the electrons more strongly towards the center. This stronger attraction pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
2. Down a Group (From Top to Bottom):
As we move down a group, the atomic radius generally increases. This occurs because the number of electron shells increases, meaning the outermost electrons are located further away from the nucleus. This increase in the distance between the nucleus and the outermost electrons leads to a larger atomic radius.
Illustrative Examples: Understanding Atomic Radius in Action
To grasp the concepts of atomic radius and its trends, let’s examine some real-world examples:
Example 1: Lithium vs. Beryllium:
Lithium (Li) and Beryllium (Be) are both located in the second period of the periodic table. Lithium, being further to the left, has a larger atomic radius than Beryllium. This is because Lithium has only one proton in its nucleus, while Beryllium has two, resulting in a stronger pull on the electrons and a smaller atomic radius for Beryllium.
Example 2: Potassium vs. Rubidium:
Potassium (K) and Rubidium (Rb) are both in the first group (alkali metals). Potassium is located above Rubidium in the periodic table. As we move down the group, the atomic radius increases. Rubidium, being further down the group, has a larger atomic radius due to the addition of an extra electron shell.
Worksheet Solutions: Mastering the Atomic Radius Puzzle
Now, let’s put our newfound knowledge to the test by tackling some common worksheet questions about atomic radius:
Question 1: Which of the following elements has the largest atomic radius: Oxygen (O), Nitrogen (N), Fluorine (F), or Neon (Ne)?
Answer: Nitrogen (N) has the largest atomic radius. All four elements are in the second period, but nitrogen is furthest to the left. As we move to the left across a period, atomic radius increases. Therefore, Nitrogen will have the largest atomic radius.
Question 2: Rank the following elements in order of increasing atomic radius: Sodium (Na), Magnesium (Mg), Aluminum (Al), and Silicon (Si).
Answer: The increasing order of atomic radius is: Silicon (Si), Aluminum (Al), Magnesium (Mg), and Sodium (Na). All four elements are in the third period, but sodium is furthest to the left. As we move to the left across a period, atomic radius increases. Therefore, sodium will have the largest atomic radius, followed by magnesium, aluminum, and silicon.
Question 3: Which of the following elements has a larger atomic radius than Chlorine (Cl): Bromine (Br), Iodine (I), or Potassium (K)?
Answer: Both Bromine (Br) and Iodine (I) have larger atomic radii than Chlorine (Cl). Bromine and Iodine are in the same group as Chlorine, but further down. As we move down a group, atomic radius increases. Potassium (K), while in the first group, is located in a different period and has a larger atomic radius than Chlorine.
Beyond Worksheets: Real-World Applications of Atomic Radius
The concept of atomic radius is not just confined to textbooks and worksheets. It has numerous real-world applications, impacting various fields from material science to medicine:
1. Material Science:
Atomic radius plays a critical role in material science, influencing the properties of different materials. For example, metals with larger atomic radii tend to be more conductive, while metals with smaller atomic radii are often stronger and more resistant to deformation.
2. Chemistry:
Atomic radius is crucial for understanding chemical bonding and reactivity. Elements with smaller atomic radii tend to form stronger bonds, while elements with larger atomic radii are more likely to participate in reactions that involve electron transfer.
3. Medicine:
Understanding atomic radius is essential in the fields of medicine and drug development. For instance, the size of atoms and molecules can influence their ability to penetrate cell membranes and interact with specific biological targets, ultimately determining the effectiveness of a drug.
Periodic Trends Atomic Radius Worksheet Answers
Conclusion: Unlocking the Secrets of Atomic Radius
This comprehensive guide has provided you with the essential information about atomic radius, its periodic trends, and applications in various fields. By understanding this fundamental concept, we can unlock a deeper comprehension of the chemical properties of elements and their significance in shaping the world around us. So, the next time you encounter a question about atomic radius, remember the insights from this guide and confidently unravel the secrets of these fascinating atomic constituents.
We encourage you to delve further into the world of atomic radius by exploring additional resources, conducting experiments, and actively engaging with the concept. The journey of understanding chemistry is never-ending, and with each new discovery, we gain a more profound appreciation for the intricate workings of the natural world.




