Have you ever wondered what makes an element an element? What makes hydrogen different from helium, or carbon different from oxygen? The answer lies in the very core of the atom: the nucleus. Within this tiny, dense center, protons and neutrons dance, dictating the element’s identity and influencing its behavior. One crucial property determined by this nuclear dance is atomic weight, a value that plays a vital role in understanding the nature of matter and its interactions.

Image: mungfali.com
In this exploration, we’ll embark on a journey through the periodic table, focusing on the first 20 elements and uncovering the fascinating story behind their atomic weights. We’ll delve into the history of atomic weight determination, understand the fundamental concepts, and discover how these values impact our lives in everyday applications.
Understanding Atomic Weight: The Foundation of Elements
A Journey into the Nucleus: Protons, Neutrons, and Isotopes
At the heart of every atom lies the nucleus, a densely packed region composed of protons and neutrons. Protons carry a positive charge and define the element’s identity, while neutrons, with no charge, contribute to the atom’s mass. The atomic number of an element, representing the number of protons, dictates its unique place on the periodic table.
However, the story doesn’t end there. Atoms of the same element can have different numbers of neutrons, giving rise to isotopes. These variations in neutron count impact the atom’s mass, leading to different atomic masses for the same element.
Atomic Weight: The Weighted Average of Isotopes
Atomic weight is not a fixed number for an element but a weighted average of the masses of its naturally occurring isotopes. This average takes into account the abundance of each isotope, reflecting the natural distribution of these variations.
The atomic mass unit (amu), approximately equal to the mass of a proton or neutron, serves as the standard unit for measuring atomic weight.
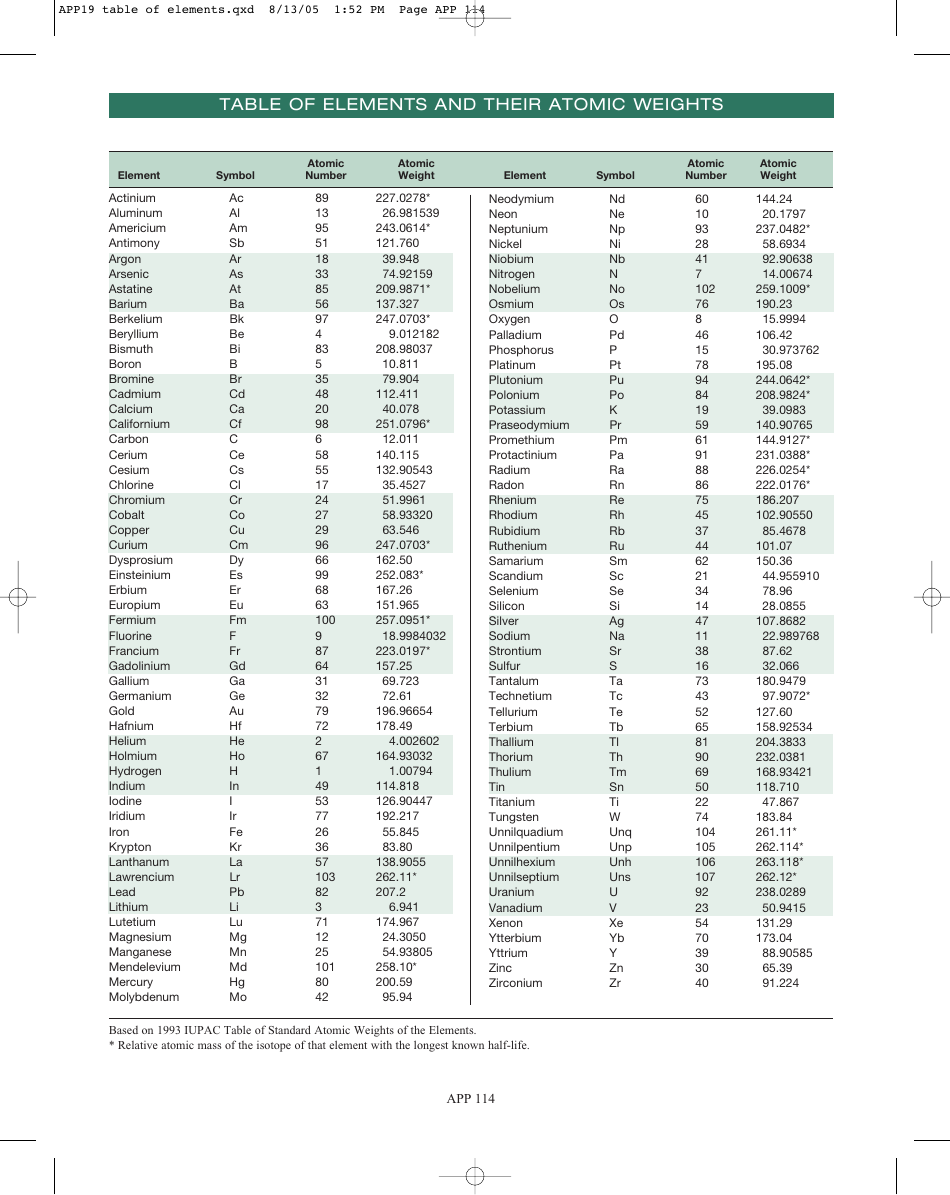
Image: www.templateroller.com
Exploring the Atomic Weights of the First 20 Elements
We can embark on a journey through the periodic table, exploring the first 20 elements and their atomic weights:
| Element | Symbol | Atomic Number | Atomic Weight (amu) |
|---|---|---|---|
| Hydrogen | H | 1 | 1.00794 |
| Helium | He | 2 | 4.002602 |
| Lithium | Li | 3 | 6.941 |
| Beryllium | Be | 4 | 9.012182 |
| Boron | B | 5 | 10.811 |
| Carbon | C | 6 | 12.0107 |
| Nitrogen | N | 7 | 14.0067 |
| Oxygen | O | 8 | 15.9994 |
| Fluorine | F | 9 | 18.9984032 |
| Neon | Ne | 10 | 20.1797 |
| Sodium | Na | 11 | 22.98976928 |
| Magnesium | Mg | 12 | 24.305 |
| Aluminum | Al | 13 | 26.9815386 |
| Silicon | Si | 14 | 28.0855 |
| Phosphorus | P | 15 | 30.973762 |
| Sulfur | S | 16 | 32.065 |
| Chlorine | Cl | 17 | 35.453 |
| Argon | Ar | 18 | 39.948 |
| Potassium | K | 19 | 39.0983 |
| Calcium | Ca | 20 | 40.078 |
Trends and Patterns: A Glimpse into Periodic Behavior
As we navigate this list, certain trends emerge, revealing the fascinating relationships between elements and their atomic weights. For instance, we observe a general increase in atomic weight as we move down the periodic table. This trend is rooted in the increasing number of protons and neutrons within the atoms of heavier elements.
The periodic table also reveals periodic changes in atomic weight as we move across a period. While the increase in atomic number (number of protons) is consistent, the addition of neutrons can vary, causing fluctuations in atomic weight.
The Importance of Atomic Weight: Insights and Applications
Understanding Chemical Reactions and Molecular Structure
Atomic weight plays a pivotal role in understanding the behavior of elements in chemical reactions. Chemical reactions involve the breaking and forming of bonds between atoms, and atomic weight influences the ratios in which elements combine.
For instance, the atomic weight of oxygen is crucial in calculating the mass of a water molecule, which contains two hydrogen atoms and one oxygen atom (H2O).
Analytical Chemistry: Determining Concentrations and Purity
Atomic weight is indispensable in analytical chemistry:
- Quantitative Analysis: Scientists use atomic weight to determine the concentration of elements in various samples, such as water, soil, and biological materials.
- Purity Assessment: Atomic weight serves as a benchmark for assessing the purity of materials, ensuring quality control in various industries.
Nuclear Chemistry: Exploring Isotopes and Radioactive Decay
Atomic weight is particularly relevant in nuclear chemistry, where isotopes and radioactive decay are central concepts.
- Isotope Analysis: Scientists study isotopes to understand the processes behind radioactive decay, dating ancient artifacts, and monitoring environmental changes.
- Radioactive Isotopes: Radioactive isotopes, with their unique decay patterns, play a crucial role in medical imaging, treatment, and scientific research.
Looking Ahead: The Future of Atomic Weight Determination
The determination of atomic weights has evolved over time, from early approximations to highly precise measurements using advanced techniques like mass spectrometry. As technology progresses, we can expect even more accurate and refined measurements of atomic weights, leading to a deeper understanding of the elements and their behavior.
Moreover, the study of atomic weights continues to generate exciting discoveries in various scientific fields. Ongoing research explores the role of atomic weight in areas such as:
- Astrophysics: The determination of atomic weights in stars and other celestial objects provides valuable insights into the evolution and composition of the universe.
- Materials Science: Understanding atomic weights is crucial for the development of new materials with tailored properties, impacting fields like electronics, energy, and medicine.
Atomic Weight Of First 20 Elements
Conclusion: From Nucleus to Everyday Life – A Journey with Atomic Weight
From the tiny nucleus to everyday applications, atomic weight plays a fundamental role in shaping our understanding of the universe around us. By delving into the atomic weights of the first 20 elements, we have glimpsed the interconnectedness of the periodic table and the underlying principles that govern the behavior of matter. This journey highlights the ongoing quest for deeper knowledge and the potential for atomic weights to drive innovation across diverse scientific frontiers.
We encourage you to continue exploring the world of atomic weights, delving into the fascinating history of their discovery, the ongoing pursuit of precision in their determination, and the exciting applications they hold for the future.




