Every time I see a bottle of cleaning solution with a pH warning label, I’m reminded of the intricate world of acidity and alkalinity. It’s not just about how strong a cleaning solution is, but about the delicate balance of hydrogen ions in our environment. In the grand scheme of things, the pH scale is more than just a number; it’s a powerful tool for understanding the world around us, from the acidity of our stomach to the alkalinity of our soil. And while it may seem daunting, understanding the pH scale is truly a key to unlocking a deeper understanding of chemistry, biology, and even our own health.
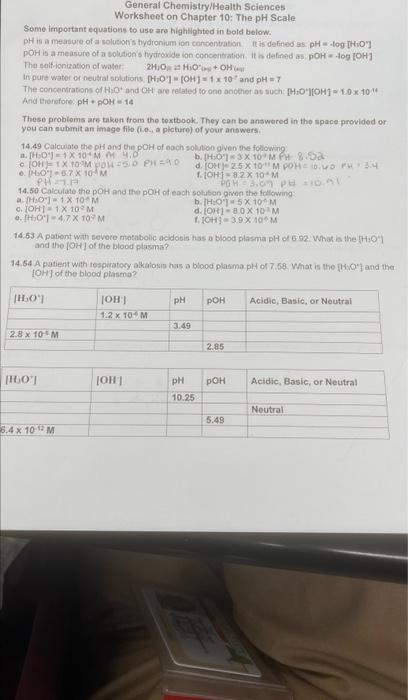
Image: www.chegg.com
In this article, we’ll embark on a journey to uncover the secrets of the pH scale, delving into its history, its influence on our lives, and how we can leverage its power. Whether you’re a curious individual seeking to understand the world around you or a student navigating the complexities of chemistry, this exploration will serve as your guide to the multifaceted concept of pH.
Understanding the pH Scale: A Journey Through Acidity and Alkalinity
The pH scale, a numerical scale ranging from 0 to 14, quantifies the acidity or alkalinity of a solution. A solution’s pH is determined by the concentration of hydrogen ions (H+) present. The higher the concentration of hydrogen ions, the more acidic the solution, and vice versa. Think of it as a balance – when hydrogen ions are numerous, the solution is acidic, while a low concentration of hydrogen ions signals an alkaline solution.
The pH Scale: A Spectrum of Acidity and Alkalinity
The pH scale is a logarithmic scale, meaning that each step represents a tenfold change in the concentration of hydrogen ions. This means that a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and a hundred times more acidic than a solution with a pH of 5.
Here’s a breakdown of where different substances fall on the pH scale:
- 0-3: Highly acidic (battery acid, stomach acid)
- 4-6: Acidic (lemon juice, vinegar)
- 7: Neutral (pure water)
- 8-10: Alkaline (baking soda, seawater)
- 11-14: Highly alkaline (bleach, ammonia)
Exploring the pH Scale’s Applications: From Everyday Life to Scientific Research

Image: worksheetlibbarth.z19.web.core.windows.net
Everyday Applications of the pH Scale
The pH scale is present in numerous aspects of our daily lives, often working silently behind the scenes. Here are a few examples:
- Food and Beverages: The taste and acidity of food and beverages are directly related to their pH. For example, citrus fruits like lemons have a pH of about 2, while milk has a pH of about 6.5.
- Personal Care Products: Shampoos, conditioners, and soaps are formulated to achieve specific pH levels to match the natural pH of our skin and hair.
- Cleaning: Strong cleaners often have a high pH to break down dirt and grime, while mild cleaners have a lower pH, making them suitable for delicate surfaces.
- Gardening: The pH of soil greatly influences plant growth, with different plants thriving in specific pH ranges.
- Pool Maintenance: Maintaining the correct pH level in swimming pools is essential for preventing skin irritation and keeping the water clear.
The pH Scale in Science and Research
Beyond everyday applications, the pH scale plays a crucial role in various scientific disciplines:
- Chemistry: The pH scale is fundamental to understanding acid-base reactions, which are crucial for many chemical processes.
- Biology: The pH of bodily fluids is vital for maintaining homeostasis, and deviations from the optimal pH can indicate health issues.
- Environmental Science: The pH of water bodies is an important indicator of environmental health. Acid rain, for instance, is a major environmental concern that can significantly alter the pH of lakes and streams.
Using pH test kits or pH meters, scientists, researchers, and even everyday individuals can measure the pH of various solutions and substances, providing valuable insights into different aspects of our world.
Navigating the pH Scale: Tips and Expert Advice
Understanding the pH scale can empower you to make informed decisions about the products you use and the environment you interact with. Here are some tips and expert advice for navigating the pH landscape:
1. Be Mindful of pH in Personal Care Products
Opt for products with a pH close to your skin’s natural pH (about 5.5) to avoid irritation, dryness, and other skin problems. Look for products labeled “pH-balanced.”
2. Understanding Soil pH and Gardening
Invest in a soil testing kit to determine the pH of your garden soil. Adjusting soil pH through the use of amendments like lime or sulfur can enhance the growth of your plants.
3. Taking Care of Your Swimming Pool
Maintaining the pH levels in your swimming pool is essential for both health and aesthetics. Invest in a pool testing kit and regularly check the pH levels, making adjustments as needed.
4. Understanding the Impact of pH on Health
While a neutral pH is generally considered optimal for bodily fluids, maintaining a slightly alkaline pH may be beneficial for overall health. Consuming alkaline-rich foods, such as fruits, vegetables, and nuts, could help support this balance.
FAQs About the pH Scale
Q: What is the difference between pH and pOH?
While pH measures the concentration of hydrogen ions (H+), pOH measures the concentration of hydroxide ions (OH-). In essence, they are complementary scales, with pH measuring acidity and pOH measuring alkalinity. The sum of pH and pOH values for a solution at a given temperature will always be equal to 14.
Q: How do I measure the pH of a solution?
There are several methods for measuring pH. The easiest and most common way is by using pH test strips or paper, which react with the solution to show a color change indicating a specific pH range. More precise measurements can be obtained with a pH meter, a digital device that directly measures the pH of a solution.
Q: What are some examples of pH indicators?
pH indicators are substances that change color depending on the pH of a solution. Some commonly used indicators are:
- Litmus paper: turns red in acidic solutions and blue in alkaline solutions.
- Phenolphthalein: colorless in acidic solutions and pink in alkaline solutions.
- Methyl orange: red in acidic solutions and yellow in alkaline solutions.
Q: Can I change the pH of a solution?
Yes, the pH of a solution can be changed by adding acids or bases. Adding an acid will lower the pH, making the solution more acidic. Adding a base will increase the pH, making the solution more alkaline.
Investigating The Ph Scale Answer Key
Conclusion: Embracing the pH Scale’s Insights
The pH scale, with its simple yet profound insights, offers a window into the intricate world of acidity and alkalinity. From understanding the chemistry of our environment to maintaining our health, embracing the pH concept can empower us to make informed decisions about the products we use and the choices we make.
Do you find the pH scale fascinating? Have you ever used a pH test kit or a pH meter? Share your thoughts and experiences in the comments below!




