Have you ever wondered how we can determine the exact amount of an unknown substance in a solution? It’s a question that has perplexed scientists for centuries, leading to the development of powerful analytical techniques like titration. In this post, we’ll embark on a journey into the fascinating world of acid-base titrations, exploring the essential principles and practical applications of this crucial laboratory technique.
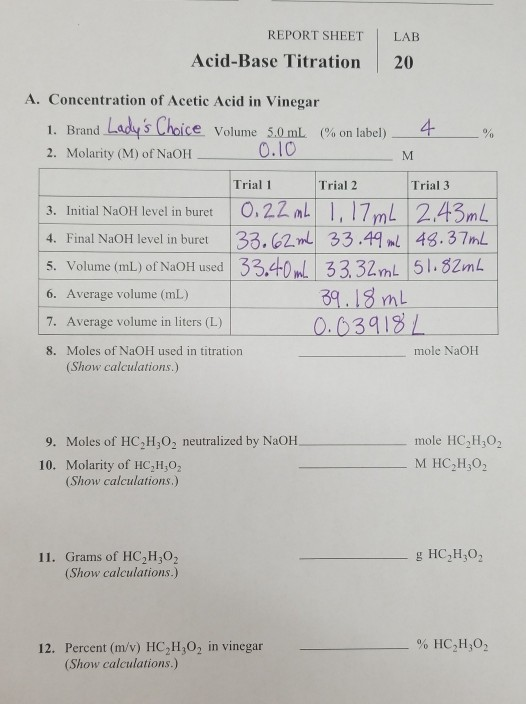
Image: melanie-chapter.blogspot.com
Imagine you’re a chemist tasked with creating a specific solution for a medical treatment or industrial process. You need to know the precise concentration of a substance to ensure the solution’s effectiveness. That’s where titration comes in – a meticulous process that allows us to determine the unknown concentration of a solution by reacting it with a solution of known concentration.
Understanding the Fundamentals of Titration
At its core, titration is a volumetric analysis technique that relies on the careful and controlled reaction between two solutions. One solution, called the analyte, is the unknown substance whose concentration we want to find. The other solution, called the titrant, has a known concentration and is added gradually to the analyte until the reaction is complete.
The reaction between the analyte and titrant is monitored using an indicator, a substance that changes color at a specific point in the reaction known as the equivalence point. This point signifies that the two solutions have reacted completely, and we can then use the volume of titrant consumed to calculate the concentration of the analyte.
The Magic of Acid-Base Titration
Acid-base titrations are a specialized type of titration where an acid solution is used to neutralize a base solution, or vice-versa. The reaction between an acid and base results in the formation of salt and water. This type of titration is incredibly useful in various applications:
- Determining the concentration of unknown acids or bases: Whether it’s a laboratory experiment or a quality control test in a manufacturing facility, acid-base titration provides a precise method for analyzing the concentration of acidic or basic substances.
- Understanding the strength of acids and bases: The strength of an acid or base refers to its ability to donate or accept protons (H+ ions). Titration can help determine the dissociation constant (Ka or Kb) of an acid or base, providing valuable insight into its strength.
- Monitoring environmental pollution: Acid-base titrations play a crucial role in environmental monitoring, allowing scientists to assess the pH (acidity or alkalinity) of water bodies, soil, or air samples to identify potential pollution issues.
The Methodology of Titration: A Step-by-Step Guide
Let’s break down the steps involved in performing an acid-base titration:
- Preparation: Begin by carefully measuring a known volume of an analyte solution, typically using a volumetric flask. Then, fill a burette with the titrant solution, ensuring it’s at a known concentration.
- Titration: Add the titrant solution to the analyte solution drop by drop while constantly stirring the mixture. As the titrant is added, the pH of the analyte solution changes.
- Observation: Monitor the pH change using a pH meter or an indicator. The indicator will change color at the equivalence point, indicating that the reaction is complete.
- Calculation: Record the volume of titrant used to reach the equivalence point. Then, use the known concentration of the titrant and the volume of the analyte to calculate the unknown analyte concentration.

Image: www.vrogue.co
Accuracy is Key: Ensuring Reliable Results
To obtain reliable and accurate titration results, it’s crucial to follow meticulous procedures:
- Proper equipment: Use calibrated equipment like burets, pipettes, and volumetric flasks to ensure accurate measurements.
- Indicator selection: Select an appropriate indicator that will change color at the equivalence point of the specific acid-base reaction.
- Stirring: Thoroughly stir the mixture during titration to ensure a homogeneous reaction.
- Careful observation: Pay close attention to the color change of the indicator, making note of the exact volume of titrant used at the equivalence point.
Practical Examples of Titration in Action
Titration isn’t just a theoretical concept; it’s an essential tool used in diverse fields:
- Pharmaceutical industry: Titration is used to ensure the purity and concentration of drugs and pharmaceutical ingredients.
- Food science: Titration helps determine the acidity of foods and beverages, impacting their taste and shelf life.
- Environmental science: Titration plays a crucial role in monitoring water quality and assessing soil acidity, contributing to environmental protection efforts.
- Chemical industries: Titration is indispensable for quality control in chemical manufacturing, ensuring the precise concentration of chemicals in various products.
Expert Insights: Tips for Success
Dr. Emily Carter, a renowned chemist and expert in analytical techniques, shares her insights on maximizing titration accuracy:
“Always double-check your equipment and ensure everything is properly calibrated before starting the titration,” she emphasizes. “The smallest inaccuracy in measurements can significantly impact the final results.”
Dr. Carter also advises students to “practice good laboratory techniques, such as careful stirring and accurate observation, to achieve reliable results.”
Titration Of Acids And Bases Lab Report
Conclusion: Unleashing the Power of Titration
Titration is a powerful analytical tool that empowers us to determine the concentration of unknown solutions with precision and accuracy. From pharmaceutical production to environmental monitoring, this technique plays a crucial role in diverse fields, ensuring the quality and safety of products and processes. By understanding the fundamental principles and practical applications of titration, we can unlock its remarkable potential for scientific advancement and technological innovation.
Do you have any interesting experiences or insights about titration in your own field? Share them in the comments below!




