Ever wondered how many tiny atoms make up the everyday objects around you? From the water you drink to the air you breathe, everything is composed of these fundamental building blocks. Stepping into the microscopic world of atoms might seem daunting, but counting them is a skill that anyone can master. This guide delves into the fascinating realm of counting atoms, providing a comprehensive understanding of the concept and offering a valuable resource for educators and students alike.

Image: athensmutualaid.net
The “Counting Atoms Worksheet Answer Key PDF” serves as a valuable tool for both students and instructors. It acts as a guide to understanding the fundamental principles of atomic composition, providing solutions and explanations to help students grasp the concepts. This worksheet is a stepping stone towards understanding the fundamental nature of matter, paving the way for more complex concepts in chemistry and related fields.
The Basics of Counting Atoms
Counting atoms might seem like a monumental task, but it involves simple principles and a systematic approach. Imagine you have a bag of marbles, each representing an atom. To count these marbles (atoms), you need a few key tools:
1. Chemical Formula: Your Atomic Blueprint
Every molecule, the smallest unit of a compound, has a chemical formula that acts as its blueprint. This formula reveals the types of atoms present and their proportions. For example, the chemical formula for water, H2O, tells us that each water molecule contains two hydrogen atoms (H) and one oxygen atom (O). This formula is our starting point for counting atoms.
2. Avogadro’s Number: The Key to Scaling Up
Imagine trying to count every grain of sand on a beach. The task seems impossible, right? Similarly, dealing with atoms individually is impractical due to their microscopic size. This is where Avogadro’s number comes to our rescue. This number, approximately 6.022 x 1023, represents the number of atoms or molecules in one mole of a substance. A mole is simply a unit of measurement, just like a dozen is a unit of measurement for eggs. Think of it as a useful conversion factor that allows us to bridge the gap between the microscopic world of atoms and the macroscopic world we experience.
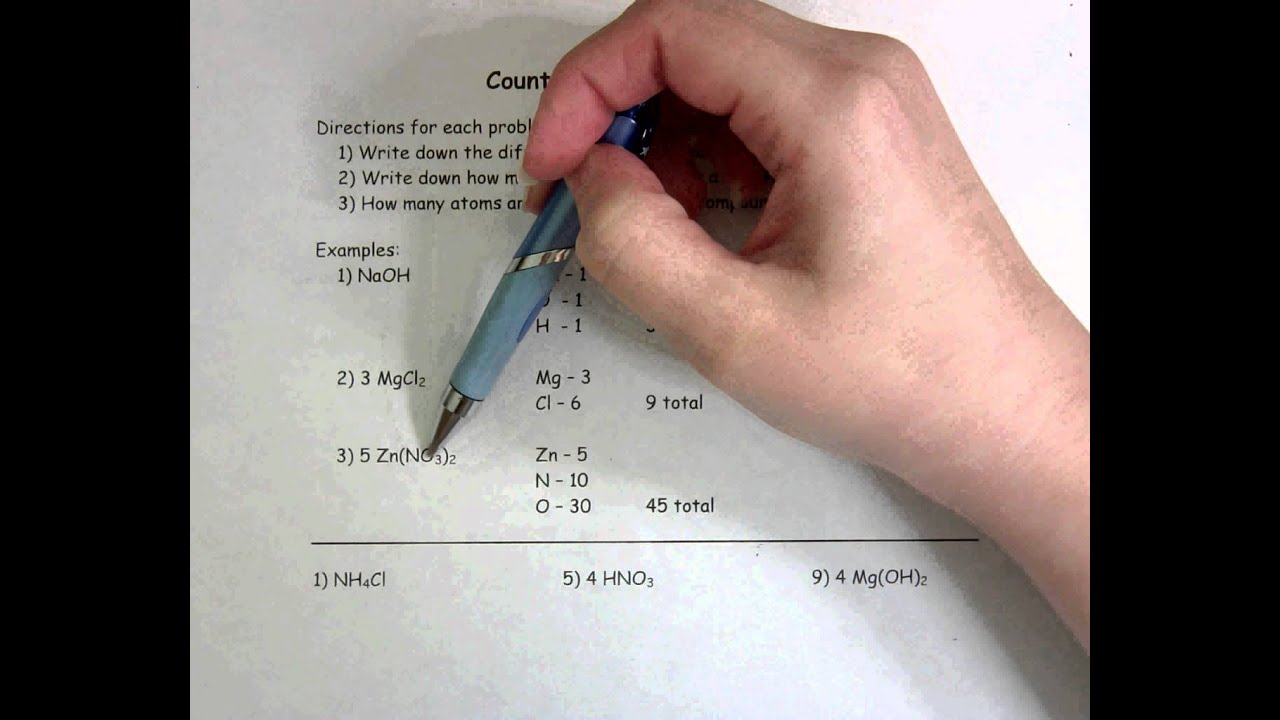
Image: www.e-streetlight.com
3. Molar Mass: Weighing in on Atomic Amounts
Every element has a unique atomic mass, a number that reflects its relative weight compared to carbon-12. The molar mass of an element is the mass of one mole of that element. For example, the molar mass of carbon is 12.01 g/mol. This means one mole of carbon weighs 12.01 grams. By knowing the molar mass of an element, we can calculate the mass of a specific number of atoms of that element.
Applying the Principles: A Step-by-Step Approach
Let’s apply these principles to a real-world example, counting the number of atoms in a sample of glucose (C6H12O6), a simple sugar that provides energy to our bodies.
1. Interpreting the Chemical Formula
From the chemical formula of glucose (C6H12O6), we see that one molecule of glucose contains 6 carbon atoms (C), 12 hydrogen atoms (H), and 6 oxygen atoms (O).
2. Finding the Molar Mass of Glucose
To calculate the molar mass of glucose, we need to sum the molar masses of each element, multiplied by their respective counts in the chemical formula:
Molar mass of glucose = (6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
3. Calculating the Number of Moles of Glucose
Let’s say you have a 36.04 gram sample of glucose. To calculate the number of moles of glucose, we divide the sample mass by the molar mass:
Number of moles of glucose = 36.04 g / 180.18 g/mol = 0.2 moles.
4. Determining the Number of Atoms
Finally, to get the number of atoms in the sample, we multiply the number of moles by Avogadro’s number:
Number of carbon atoms = 0.2 moles x 6.022 x 1023 atoms/mol x 6 = 7.226 x 1023 atoms
Number of hydrogen atoms = 0.2 moles x 6.022 x 1023 atoms/mol x 12 = 1.445 x 1024 atoms
Number of oxygen atoms = 0.2 moles x 6.022 x 1023 atoms/mol x 6 = 7.226 x 1023 atoms
The “Counting Atoms Worksheet Answer Key PDF”: Your Guide to Success
The “Counting Atoms Worksheet Answer Key PDF” enhances this process, offering detailed step-by-step solutions to a wide range of problems involving counting atoms. It provides:
- Clear explanations of the concepts involved, making the process understandable even for beginners
- Practice problems to solidify your understanding of counting atoms in various contexts
- Comprehensive answer keys to verify your work and pinpoint areas where you can improve
- Illustrative examples that showcase the application of the principles in real-world situations
Beyond the Worksheet: Applications of Counting Atoms
The ability to count atoms extends beyond the confines of chemistry textbooks. It’s a fundamental skill essential for various fields, impacting:
- Drug Development: Pharmaceutical companies rely on the accurate calculation of atoms to design and synthesize new drugs, ensuring the correct dosage and optimizing therapeutic effects.
- Materials Science: Understanding the atomic composition of materials is crucial for developing new materials with specific properties, such as strength, conductivity, and heat resistance.
- Environmental Monitoring: Determining the presence and concentrations of various atoms, such as pollutants in water or air, is vital for environmental protection and public health.
- Food Science: Counting atoms allows us to analyze the nutritional content of foods, ensuring balanced diets and understanding the roles of different elements in our bodies.
Counting Atoms Worksheet Answer Key Pdf
Conclusion
Mastering the art of counting atoms unlocks a deeper understanding of the fundamental building blocks of our world. While the “Counting Atoms Worksheet Answer Key PDF” provides a solid framework for developing this skill, the journey doesn’t end there. Continue to explore the fascinating world of atoms and its impact on our lives. This newfound knowledge will empower you to appreciate the intricate world of chemistry and its intricate role in shaping our everyday experiences.




