Remember those colorful diagrams from your high school science class, showing a tiny nucleus surrounded by whirling electrons? That was a basic depiction of the atom, the fundamental building block of all matter. While that simplified model may come to mind, there’s a whole lot more to the story of atomic structure. This is where the “Basic Atomic Structure Worksheet Key 2” comes in, offering a deeper dive into the fascinating world of atoms and how they’re organized.
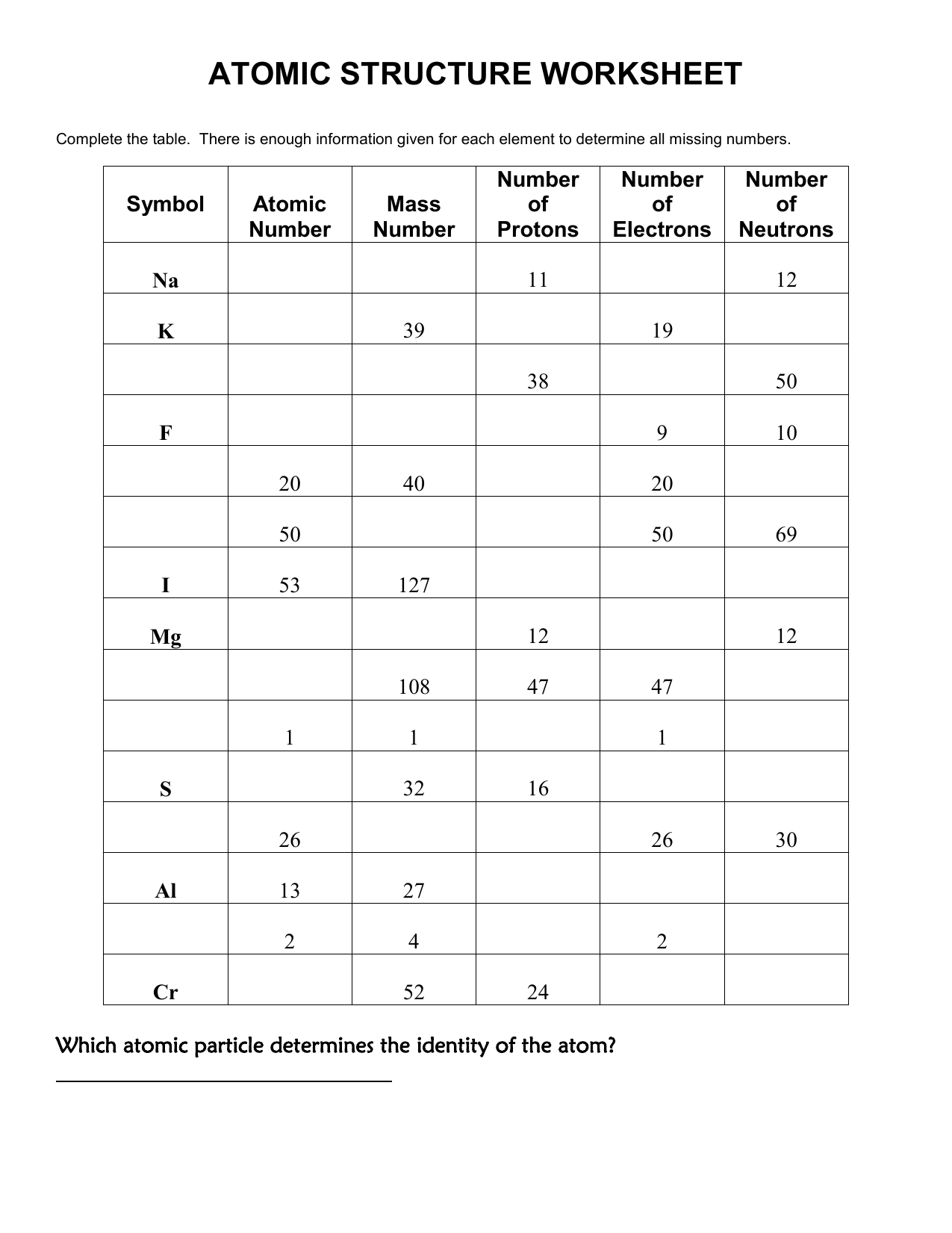
Image: answerschoolschafer.z13.web.core.windows.net
Recently, I stumbled upon an old notebook filled with my high school science notes. Flipping through those pages, I was surprised to rediscover a worksheet titled “Basic Atomic Structure Worksheet Key 2.” This worksheet was my entry point into understanding atomic structure. It’s the kind of worksheet that helps you grasp the fundamentals, those key concepts that form the foundation for further exploration. It’s not just about memorizing facts; it’s about developing a sense of how atoms work.
Unlocking the Secrets of Atoms with Worksheets
We all remember the basic model of an atom: a tiny, dense nucleus at the center, containing protons and neutrons, surrounded by a cloud of negatively charged electrons. But that’s just the tip of the iceberg. The “Basic Atomic Structure Worksheet Key 2” takes us beyond this simple picture, delving into the intricacies of isotopes, electron configurations, and the periodic table’s role in organizing elements.
These worksheets often employ a variety of activities to help students master atomic structure. You might find diagrams to label, questions to answer, or even simulations to interact with. The goal is to turn passive learning into an active, engaging process. Worksheets like this provide a structured framework for organizing information, making it easier to understand and remember.
Understanding the Basics: A Deeper Look
Atomic Structure: The Foundation of Chemistry
The atomic structure is the foundation of chemistry. It lays the groundwork for understanding how atoms bond with each other to form molecules, how elements behave in chemical reactions, and how different substances interact. Mastering the concepts of atomic structure is essential for comprehending the vast world of chemical reactions and the properties of matter.
Here’s a quick rundown of the key components:
- Protons: Positively charged particles located in the nucleus. The number of protons determines the element.
- Neutrons: Neutral particles found in the nucleus. They contribute to the atom’s mass.
- Electrons: Negatively charged particles that orbit the nucleus in energy levels or shells. Electron configurations determine how atoms interact with each other.

Image: www.pinterest.com
Isotopes: Variations in Atomic Structure
Atoms of the same element can have different numbers of neutrons. These variations are known as isotopes. For example, carbon-12 and carbon-14 are isotopes of carbon. Carbon-12 has 6 protons and 6 neutrons while carbon-14 has 6 protons and 8 neutrons. These variations have implications for chemical behavior and radioactive decay.
Electron Configurations: Deciphering Atomic Behavior
Electron configurations describe the arrangement of electrons within an atom’s energy levels. These configurations provide insights into an atom’s reactivity and bonding properties. Understanding electron configurations helps us predict how atoms will bond to form molecules and how they will participate in chemical reactions.
The Periodic Table: Organizing the Elements
The periodic table is essentially a map of the elements, arranged by increasing atomic number. The periodic table reveals patterns in elements’ properties and helps us predict their behavior. It’s a powerful tool for understanding the relationships between elements and for predicting the properties of unknown elements.
Learning by Doing: Tips for Mastering Atomic Structure
Learning about atomic structure can be like navigating a complex maze. Here are some tips to make your journey smoother and more rewarding:
- Visualize it: Use diagrams and models to help you visualize the arrangement of protons, neutrons, and electrons within an atom.
- Practice, practice, practice: Work through as many practice problems and worksheets as possible. Repetition reinforces understanding.
- Don’t be afraid to ask questions: If you encounter a concept that you don’t understand, don’t hesitate to seek help from your teacher, tutor, or classmates.
- Connect the concepts: Don’t treat each piece of information in isolation. Think about how different concepts relate to each other.
- Look for patterns: The periodic table is a great resource for discovering patterns in the properties of elements.
Atomic Structure in Action: Beyond the Textbook
Atomic structure isn’t just a topic confined to textbooks. It’s at the heart of many real-world applications:
- Nuclear Power: The generation of electricity through nuclear reactions is based on understanding atomic structure and how to manipulate nuclear forces.
- Medical Imaging: Techniques like PET scans and MRI use atomic properties to create detailed images of the human body.
- Materials Science: The development of new materials with specific properties (strength, conductivity, etc.) relies heavily on atomic structure principles.
Frequently Asked Questions
Q: What is the difference between an atom and a molecule?
A: An atom is the basic building block of matter, while a molecule is formed when two or more atoms bond together.
Q: How do you determine the number of neutrons in an atom?
A: Subtract the atomic number (number of protons) from the atomic mass number (total number of protons and neutrons) to get the number of neutrons.
Q: What are the different types of chemical bonds?
A: Common types include ionic bonds (transfer of electrons), covalent bonds (sharing of electrons), and metallic bonds (free movement of electrons).
Q: How does understanding atomic structure help us in daily life?
A: It helps us understand the properties of materials we use every day, from the plastics in our phones to the metals in our cars.
Basic Atomic Structure Worksheet Key 2
Conclusion
The “Basic Atomic Structure Worksheet Key 2” serves as a valuable guide to unlocking the mysteries of atoms. These worksheets are designed to engage students and help them master the fundamentals of atomic structure – a topic that forms the base for understanding all of chemistry and many other scientific disciplines. So, if you’re looking to delve deeper into the fascinating world of atoms, don’t hesitate to take advantage of the incredible resources available to you.
Are you curious to learn more about atomic structure and its applications? Share your thoughts in the comments below!




