Have you ever wondered how a seemingly simple act, like adding a piece of metal to a solution, can unleash a cascade of chemical reactions? Or how we can determine the precise composition of a substance, down to the level of its constituent elements? In the realm of chemistry, the answers lie in the fascinating world of replacement reactions and percent composition. This Chem 152 lab 6 delves into these concepts, providing a hands-on exploration of these foundational chemical principles.
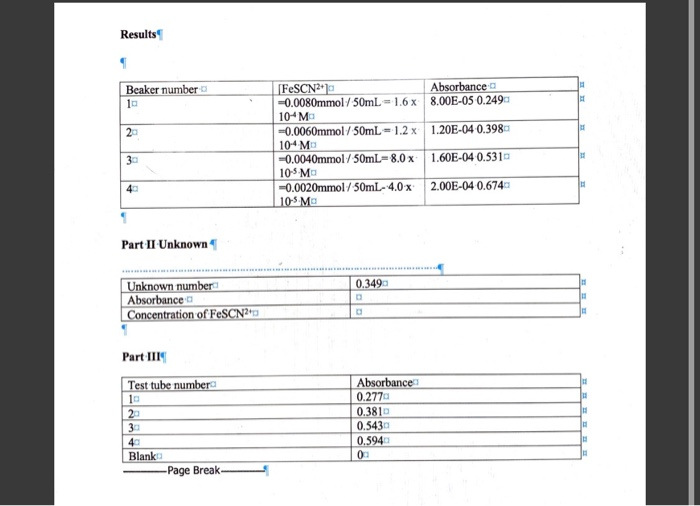
Image: www.chegg.com
This lab is a journey of discovery, guiding you through a series of experiments designed to reveal the intricacies of replacement reactions and percent composition. From the vibrant colors of chemical transformations to the careful calculations that reveal elemental proportions, this lab offers a unique opportunity to witness chemistry in action. Let’s embark on this exciting expedition, starting with a deep dive into the heart of replacement reactions and percent composition.
Understanding the Foundations of Replacement Reactions
Replacement reactions, also known as single displacement reactions, are fundamental chemical processes that form the basis of many natural and industrial processes. At the core of replacement reactions lies the concept of reactivity: the inherent tendency of elements to lose or gain electrons, a key driver of chemical change.
Imagine a scenario where a more reactive element, eager to give up its electrons, encounters a less reactive element holding onto its electrons. In such a scenario, the more reactive element will “kick out” the less reactive element, taking its place in the compound. This transfer of electrons, accompanied by the rearrangement of atoms, constitutes a replacement reaction.
Deciphering the Language of Chemical Equations
To understand the intricacies of replacement reactions, we must first master the language of chemical equations. These equations are like chemical recipes, providing a concise and symbolic representation of the reactants (starting materials) and products (formed substances) involved in a reaction.
A typical replacement reaction equation might look like this:
A + BC → AC + B
In this equation, element “A” is more reactive than element “B”. “A” replaces “B” in the compound “BC”, resulting in the formation of “AC” and the release of “B”.
The Importance of Reactivity Series
To predict if a replacement reaction will occur, chemists rely on the reactivity series, a valuable tool that arranges elements in order of their decreasing reactivity. Elements higher on the reactivity series are more likely to displace elements lower on the series.
For example, if we consider the following reaction:
Zn + CuSO4 → ZnSO4 + Cu
Zinc (Zn) is higher on the reactivity series than Copper (Cu). Therefore, zinc will displace copper from copper sulfate (CuSO4), forming zinc sulfate (ZnSO4) and releasing copper.
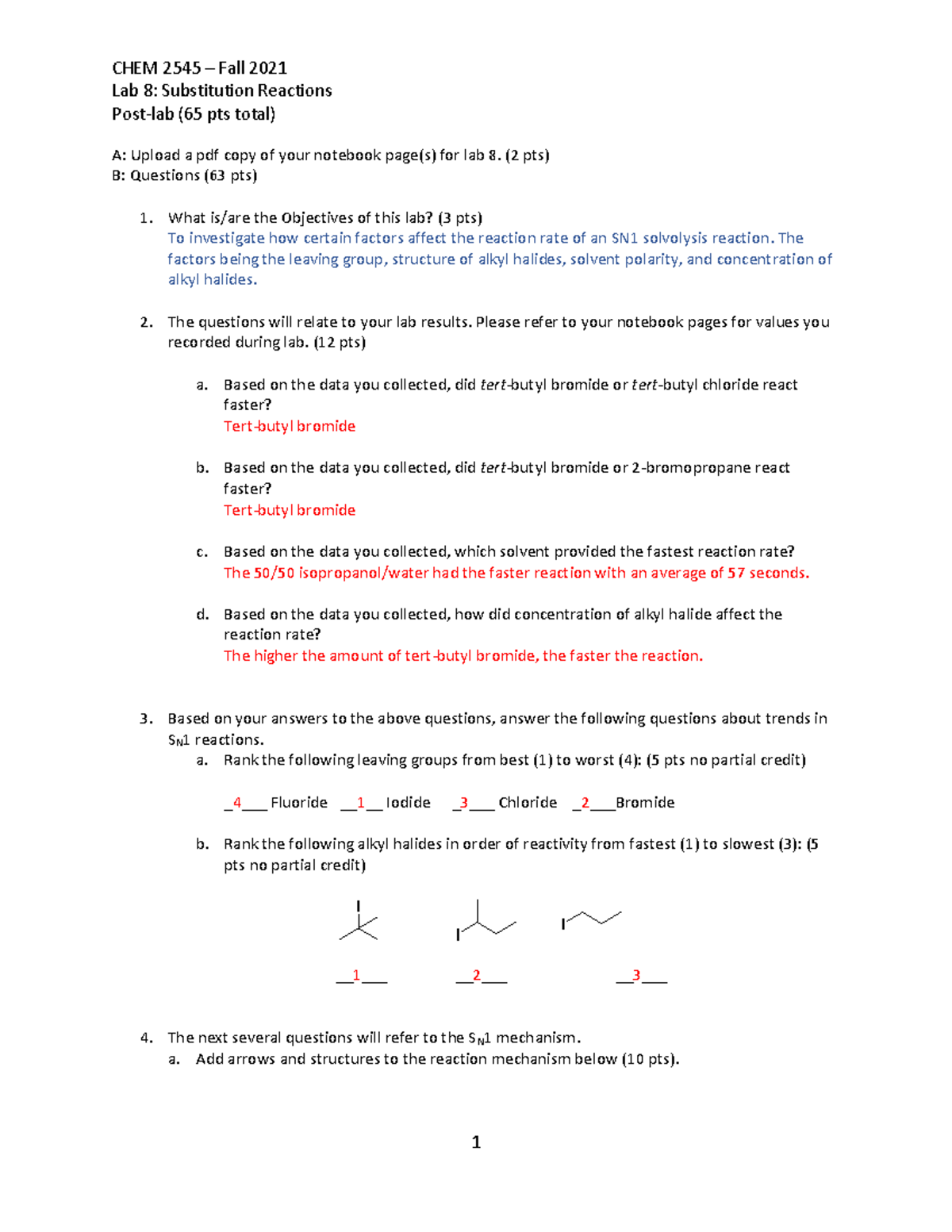
Image: www.studocu.com
The Intricacies of Percent Composition
The concept of percent composition extends beyond reactions, offering a fundamental understanding of the relative proportions of elements within a compound. Imagine a molecule like water, H2O. Percent composition tells us the percentage of hydrogen and oxygen by mass that make up this molecule. This information is crucial in countless applications, from analyzing chemical substances to understanding the composition of materials in our daily lives.
To determine percent composition, we employ the following formula:
Percent Composition = (Mass of Element / Mass of Compound) x 100%
Let’s illustrate this concept with a simple example:
Consider a compound with the formula NaCl, sodium chloride. The atomic mass of sodium (Na) is 22.99 amu, and the atomic mass of chlorine (Cl) is 35.45 amu. Therefore, the molar mass of NaCl is 58.44 amu.
To calculate the percent composition of sodium:
Percent Na = (22.99 amu / 58.44 amu) x 100% = 39.34%
Similarly, the percent composition of chlorine:
Percent Cl = (35.45 amu / 58.44 amu) x 100% = 60.66%
This calculation tells us that sodium chloride is made up of approximately 39.34% sodium and 60.66% chlorine by mass.
Chem 152 Lab 6: A Practical Exploration
In Chem 152 Lab 6, students engage in a series of hands-on experiments designed to solidify their understanding of replacement reactions and percent composition.
- Experiment 1: Investigating Metal Reactivity
In this experiment, students will observe the reactions between various metals and solutions containing different cations. By analyzing the reactions and their products, they will be able to determine the relative reactivity of different metals and create a reactivity series. This experiment allows for a visual and practical confirmation of the reactivity series, providing concrete evidence for the principles discussed earlier.
- Experiment 2: Determining the Percent Composition of a Compound
Here, students will work with a known compound and, through meticulous measurements and calculations, determine its percent composition. This experiment involves carefully weighing, dissolving, and reacting the compound to isolate and quantify its constituent elements. The process allows students to apply the principles of stoichiometry, conversions, and percent composition calculations, culminating in a precise determination of the elemental makeup of the compound.
Practical Applications of Replacement Reactions and Percent Composition
The knowledge gained from Chem 152 Lab 6 has widespread applications in various fields:
- Chemical Synthesis
Replacement reactions form the core of countless chemical synthesis processes, enabling the production of a vast array of materials, from pharmaceuticals to plastics. Understanding the reactivity of elements is essential in designing and optimizing these synthetic pathways, ensuring efficient and targeted production of desired materials.
- Environmental Remediation
Replacement reactions are critical in environmental remediation efforts. They are utilized in removing pollutants, such as heavy metals, from contaminated water and soil. By carefully selecting reactive species, environmental engineers can effectively remove these harmful contaminants, promoting a safer and healthier environment.
- Corrosion Prevention
One of the most important applications of the reactivity series lies in corrosion prevention. For example, galvanizing steel by coating it with zinc prevents rusting. We understand, based on the reactivity series, that zinc is more reactive than iron. Therefore, the zinc layer will oxidize first, sacrificing itself to protect the iron below.
- Material Characterization
Percent composition analysis is a fundamental tool in material characterization, providing a detailed understanding of the elemental makeup of various substances. This information is crucial in designing and optimizing materials for specific applications, from building structures to microelectronics.
- Forensic Science
In forensic science, percent composition analysis plays a vital role in identifying substances and tracing their origins. By determining the precise elemental composition of unknown samples, forensic experts can establish connections between evidence and suspects, contributing to the investigation and resolution of crimes.
Expert Insights for a Deeper Understanding
To further enrich your understanding of replacement reactions and percent composition, consider seeking insights from experts in the field of chemistry. Online forums, educational videos, and specialized textbooks can provide valuable information and practical examples. Engaging with these resources can deepen your comprehension and enhance your ability to apply these principles in various contexts.
Chem 152 Lab 6 Replacement Reactions And Percent Composition
Empowering Yourself with Chemical Knowledge
The knowledge gained from Chem 152 Lab 6 empowers you with a deeper understanding of the fundamental principles governing chemical reactions. It provides a solid foundation for future explorations in chemistry and enables you to confidently apply these concepts in various fields, from medicine and engineering to environmental science and forensic investigations. Embrace the journey of discovery, explore the fascinating world of chemistry, and empower yourself with the knowledge to make a positive impact!




