Have you ever wondered how scientists can accurately measure and manipulate substances so small they’re invisible to the naked eye? The answer lies in a powerful tool called the mole, a unit that bridges the microscopic world of atoms and molecules with the macroscopic world we experience. Mastering the mole concept is crucial for understanding chemical reactions and calculations, and that’s where practice problems, like those found in “mass and the mole worksheets,” come into play. This article will delve into the world of the mole, explore the answers to common worksheet problems, and demonstrate how this concept empowers us to understand the composition and behavior of matter.

Image: alekohovekamp.blogspot.com
Imagine you’re baking a cake. The recipe calls for a specific amount of each ingredient, like flour, sugar, and eggs. These ingredients are like atoms and molecules, the building blocks of matter. Just as a recipe ensures the cake turns out right, the mole concept helps us determine the precise quantities of atoms and molecules needed for chemical reactions to occur. In essence, the mole is a bridge between the microscopic world of atoms and the macroscopic world of grams and liters, making it a fundamental concept in chemistry.
Delving into the Mole: The Building Block of Chemical Calculations
The mole, often referred to as Avogadro’s number, represents a specific quantity of particles, be it atoms, molecules, or ions. This number, approximately 6.022 x 10^23, is incredibly large. To grasp its magnitude, think of a mole of sand grains – it would be enough to cover the entire Earth in a layer over 13 miles deep! This enormous number highlights the tiny scale of atoms and molecules.
Why is the mole so important? Because it allows us to relate the mass of a substance to the number of particles it contains. Understanding this relationship is crucial for a wide range of applications, from calculating the amount of reactants needed in a chemical reaction to determining the concentrations of solutions used in labs and industries.
Tackling Mass and Mole Worksheets: A Step-by-Step Guide
Mass and mole worksheets often present problems involving conversions between grams, moles, and the number of particles. These problems typically involve the following steps:
-
Identifying the Formula: Start by determining the chemical formula of the substance in question. This will tell you the molar mass (grams per mole) of the compound.
-
Applying the Mole Concept: The crucial link between mass and moles is the molar mass, which is the mass of one mole of the substance. For example, the molar mass of water (H2O) is approximately 18 grams per mole. This means that one mole of water weighs 18 grams.
-
Setting up Proportions: Most mass and mole problems involve setting up proportions using the molar mass as the conversion factor. By setting up a simple ratio, you can convert from grams to moles or vice versa.
-
Avogadro’s Number for Particle Conversion: When converting between moles and the number of particles (atoms, molecules, or ions), use Avogadro’s number as a conversion factor. Remember, one mole is equal to 6.022 x 10^23 particles.
-
Practice Makes Perfect: The best way to master mass and mole calculations is through practice. Work through multiple examples from worksheets or textbooks, and don’t hesitate to seek help if you encounter difficulties.
Example Problem: Mass and Mole Conversion
Let’s tackle a common mass and mole worksheet problem:
Question: How many grams of sodium chloride (NaCl) are present in 2.5 moles of NaCl?
Solution:
- Formula: The chemical formula of sodium chloride is NaCl.
- Molar Mass: The molar mass of NaCl is approximately 58.44 grams per mole (Na: 22.99 g/mol + Cl: 35.45 g/mol).
- Proportion: Set up a proportion using the molar mass:
- 58.44 grams NaCl / 1 mole NaCl = x grams NaCl / 2.5 moles NaCl
- Solve for x: Solving for x, we find that x = 146.1 grams NaCl.
Therefore, 2.5 moles of sodium chloride weigh 146.1 grams.
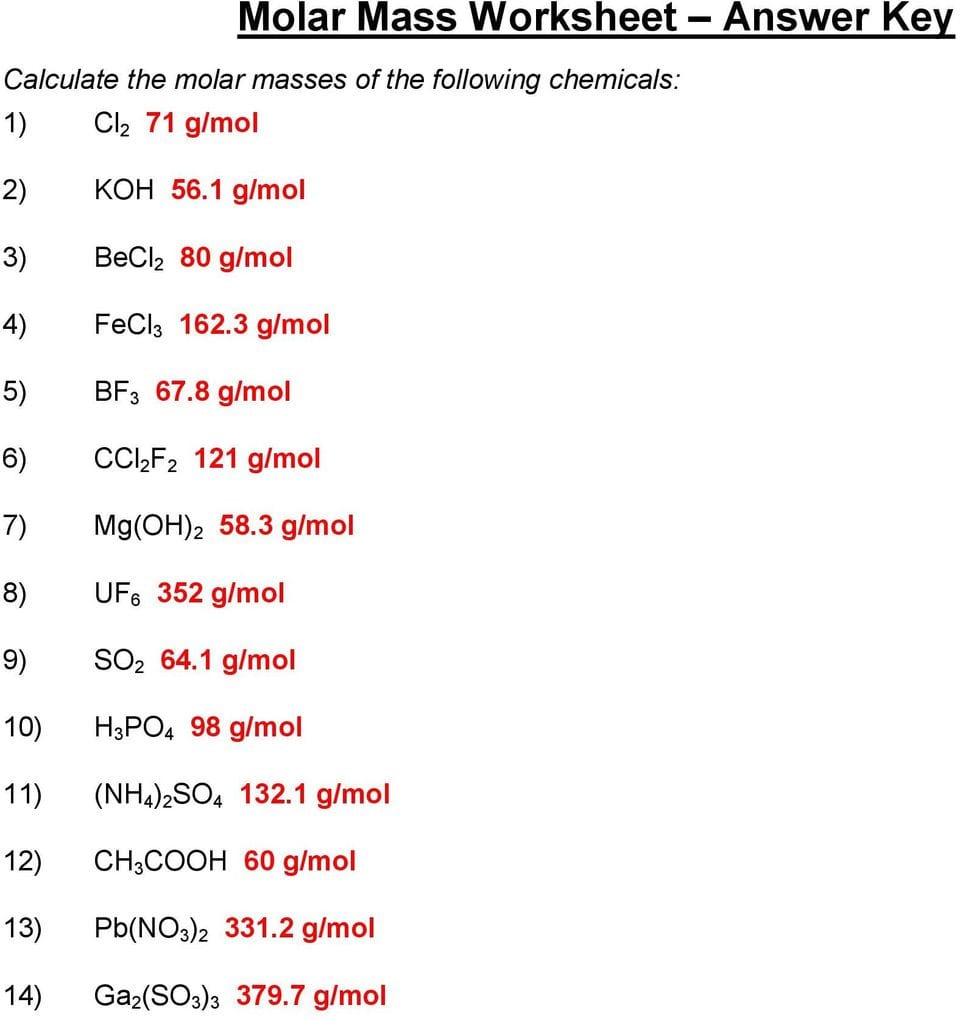
Image: db-excel.com
Expanding the Applications: From Worksheets to Everyday Chemistry
The mole concept is not just confined to textbooks and worksheets. It plays a crucial role in various real-world applications:
- Pharmaceutical Chemistry: Pharmacists use the mole concept to determine the precise dosages of medications based on the number of molecules needed for therapeutic effects.
- Industrial Chemistry: Chemical engineers rely on the mole concept to optimize production processes, ensure accurate ingredient ratios, and ensure product quality.
- Environmental Chemistry: Environmental scientists utilize the mole concept to analyze pollutants, determine their concentrations, and develop remediation strategies.
As you delve deeper into chemistry, you’ll encounter more complex scenarios involving mole calculations. Understanding the fundamentals, however, provides a solid foundation for success.
Mass And The Mole Worksheet Answers
Conclusion: Mastering the Mole, Mastering Chemistry
The mole concept is a cornerstone of chemistry, allowing us to bridge the gap between the microscopic world of atoms and the macroscopic world we interact with. By understanding how to apply the mole concept, we gain a deeper appreciation for the composition and behavior of matter. So, next time you encounter a mass and mole worksheet, remember that you are not just solving an equation, you are learning a fundamental language that unlocks the secrets of the world around us!
Further exploration:
- Consult your chemistry textbook or online resources for additional practice problems and explanations.
- Participate in online forums or discussion groups where you can interact with other students and educators.
- Remember, understanding chemistry takes time and effort, so don’t be discouraged if you face challenges along the way. With practice and determination, you will master the mole concept and unlock a deeper understanding of the wonders of chemistry!




