Have you ever wondered why some molecules are more reactive than others? Or why certain functional groups seemingly have a magnetic pull on electrons, influencing the behavior of the entire molecule? The answer lies in the fascinating world of electron withdrawing and donating groups, the unsung heroes of organic chemistry. These seemingly simple groups wield significant power, dictating the reactivity and properties of countless molecules.
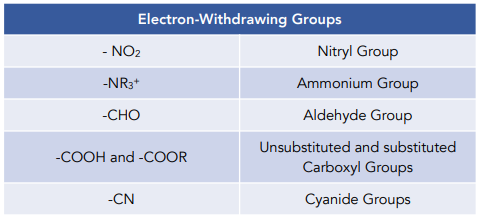
Image: notariaurbina.cl
Imagine a molecular dance floor where electrons are the dancers. Electron withdrawing groups, like the elegant waltzers, pull electrons closer to themselves, causing a localized build-up of positive charge. On the other hand, electron donating groups, like the energetic salsa dancers, push electrons away, creating a region of negative charge. This seemingly simple dance of electrons determines the fate of molecules, influencing their stability, acidity, and even their color.
Electron-Withdrawing Groups: The Magnetic Force in Molecules
Electron withdrawing groups are like the magnetic personalities on the molecular dance floor, pulling electrons towards them, creating a captivating effect on the entire molecule. They essentially “suck” electron density away from the molecule’s core, making the overall molecule more electron-deficient. This electron-deficient nature can have a profound impact on the molecule’s reactivity, making it more susceptible to nucleophilic attack.
Here are some of the most common electron-withdrawing groups you might encounter in your chemistry journey:
1. Halogens (F, Cl, Br, I):
While halogens might appear unassuming, they are masters of electron withdrawal. Their electronegativity allows them to pull electron density towards themselves, making the attached carbon more electron-deficient.
2. Nitro Group (NO₂):
This powerful group, often found in explosives, has a formidable electron-withdrawing ability. The nitrogen atom, tightly connected to two highly electronegative oxygen atoms, acts as a strong electron sink, depleting the electron density of the attached carbon.
3. Carbonyl Group (C=O):
The carbonyl group, the star of aldehydes and ketones, with its double-bonded oxygen atom, possesses a unique ability to attract electron density. The oxygen atom, being highly electronegative, pulls electron density from the carbon atom, making it electron-deficient.
4. Cyano Group (CN):
The cyano group, a stalwart of nitriles, boasts a potent electron-withdrawing capability. The nitrogen atom, linked to a carbon atom through a triple bond, effectively pulls electron density from the attached carbon, making it more reactive under nucleophilic attack.
5. Sulfonyl Group (SO₂):
Sulfonyl groups, commonly found in sulfonic acids, exhibit strong electron-withdrawing characteristics. The sulfur atom, connected to two oxygen atoms through double bonds, acts as an electron sink, making the attached carbon more susceptible to nucleophilic attack.
Electron-Donating Groups: The Energizers of Molecular Reactivity
Electron-donating groups are the energetic dancers, pushing electrons away from themselves, generating a region of negative charge. These groups, with their abundant electron supply, can influence a molecule’s reactivity, making it more likely to participate in electrophilic reactions.
Common electron-donating groups include:
1. Alkyl Groups (R-):
Alkyl groups, like the charismatic party host on the molecular dance floor, are known for their electron-donating ability. The carbon atoms in these groups, with their abundance of electrons, push electron density towards the attached carbon, increasing its electron density.
2. Hydroxyl Group (OH):
The hydroxyl group, the elegant dancer, often found in alcohols and phenols, donates electrons through its oxygen atom. The non-bonding electrons on the oxygen atom can be readily donated, increasing the electron density of the attached carbon.
3. Amino Group (NH₂):
The amino group, like a skilled choreographer in the molecular world, donates electrons through its nitrogen atom. The nitrogen atom, with its lone pair of electrons, can readily donate them, increasing the electron density of the attached carbon.
4. Ether Group (R-O-R’):
The ether group, a versatile dancer, often found in ethers and epoxides, donates electrons through its oxygen atom. The lone pair of electrons on the oxygen atom can be readily donated, increasing the electron density of the attached carbon.
5. Alkoxy Group (RO):
Like the versatile dancer, the alkoxy group, found in alcohols and ethers, donates electrons through its oxygen atom. Its lone pairs can readily donate to the attached carbon, increasing the electron density.
Understanding the Impact: Reactivity and Beyond
Now that we’ve encountered the electron withdrawing and donating groups, it’s time to appreciate their profound impact on the molecular world.
-
Acidity and Basicity: Electron-withdrawing groups increase acidity and decrease basicity by stabilizing the negative charge that results from deprotonation. The withdrawal of electrons makes the molecule more likely to give up a proton, leading to increased acidity. Conversely, electron-donating groups decrease acidity and increase basicity.
-
Reactivity: Electron-withdrawing groups increase reactivity with nucleophiles, while electron-donating groups increase reactivity with electrophiles. Molecules with electron-withdrawing groups are more susceptible to nucleophilic attack. Meanwhile, electron-donating groups make the molecule more likely to react with electrophiles.
-
Stability: Electron-withdrawing groups can stabilize carbocations, making them more stable. The withdrawal of electron density helps to offset the positive charge on the carbon, promoting stability.

Image: www.researchgate.net
Expert Insights: Unlocking the Power of Electron Withdrawing and Donating Groups
As you navigate the world of chemistry, always remember to consider the role of electron withdrawing and donating groups. They are the hidden drivers of many chemical reactions, and understanding their impact can help you predict and manipulate chemical behavior.
Key Takeaway: Electron withdrawing and donating groups play a crucial role in determining the reactivity, stability, and other properties of molecules. Their impact is widespread, affecting acid-base reactions, nucleophilic reactions, and many other chemical processes.
Electron Withdrawing And Electron Donating Groups List
Explore Further and Unleash Your Chemistry Genius
The world of electron withdrawing and donating groups is fascinating and vast. This exploration has merely scratched the surface. Continue your learning journey, delve into specific examples of these groups in action, and discover the intricate dance of electrons that governs the chemical world around us. The key is to keep exploring, questioning, and experimenting—the journey of chemical discovery awaits!




